breakthrough in
glycemic control

Tidepool Loop
The FDA-cleared algorithm developed by people with diabetes, for people with diabetes.1,2
iiSure™ Technology
The only device with sound wave technology designed to accurately measure and deliver insulin.1,3
powered by Tidepool Loop—
the opportunity for better glycemic control1*
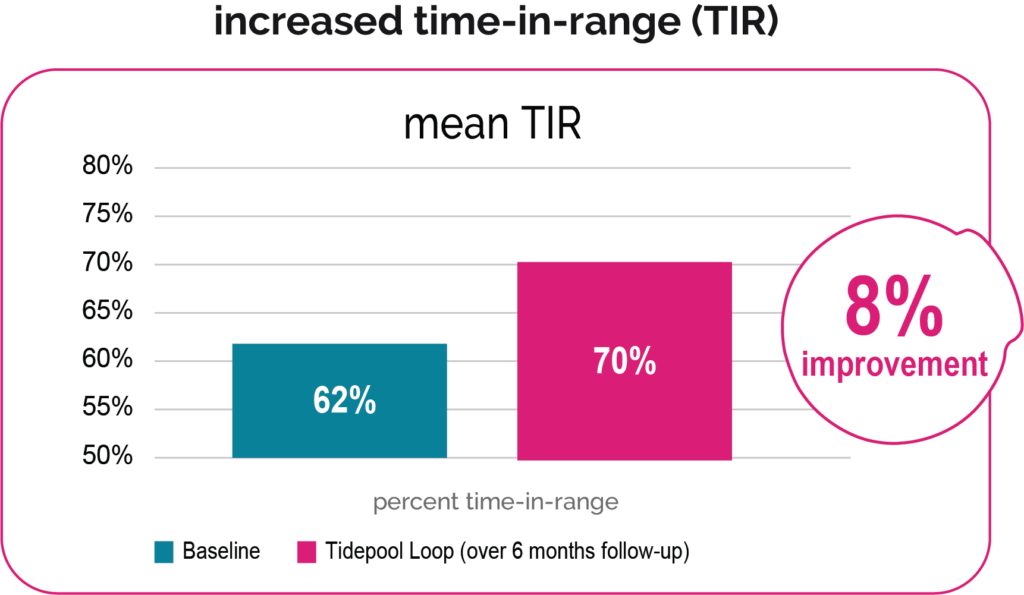
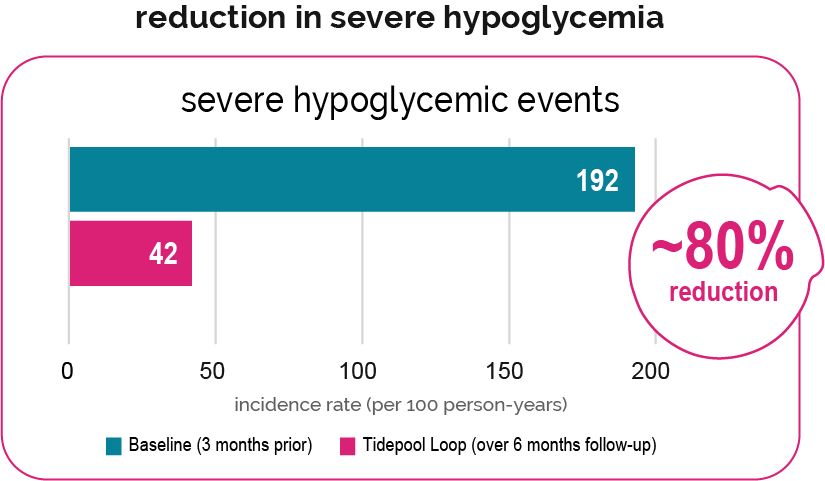
Study Design: The Jaeb Center for Health Research, in collaboration with Stanford University and Tidepool, conducted an open-enrollment, observational study to collect data on the do-it-yourself (DIY) Loop system (ClinicalTrials.gov Identifier: NCT03838900). This study collected and analyzed data on the efficacy, safety, usability, and quality of life/psychosocial effects of the DIY Loop System in patients diagnosed with type 1 diabetes (T1D) who were using insulin (pump therapy or multiple daily injections) and were using DIY Loop or had plans to begin using DIY Loop. Tidepool Loop is based on the same algorithm as DIY Loop.
Intended User Population: Study population (n=175) was limited to the intended user population (ages 6 and up, use of Humalog or Novolog, settings within allowable Tidepool Loop ranges at least 90% of the time).
supporting real-world evidence
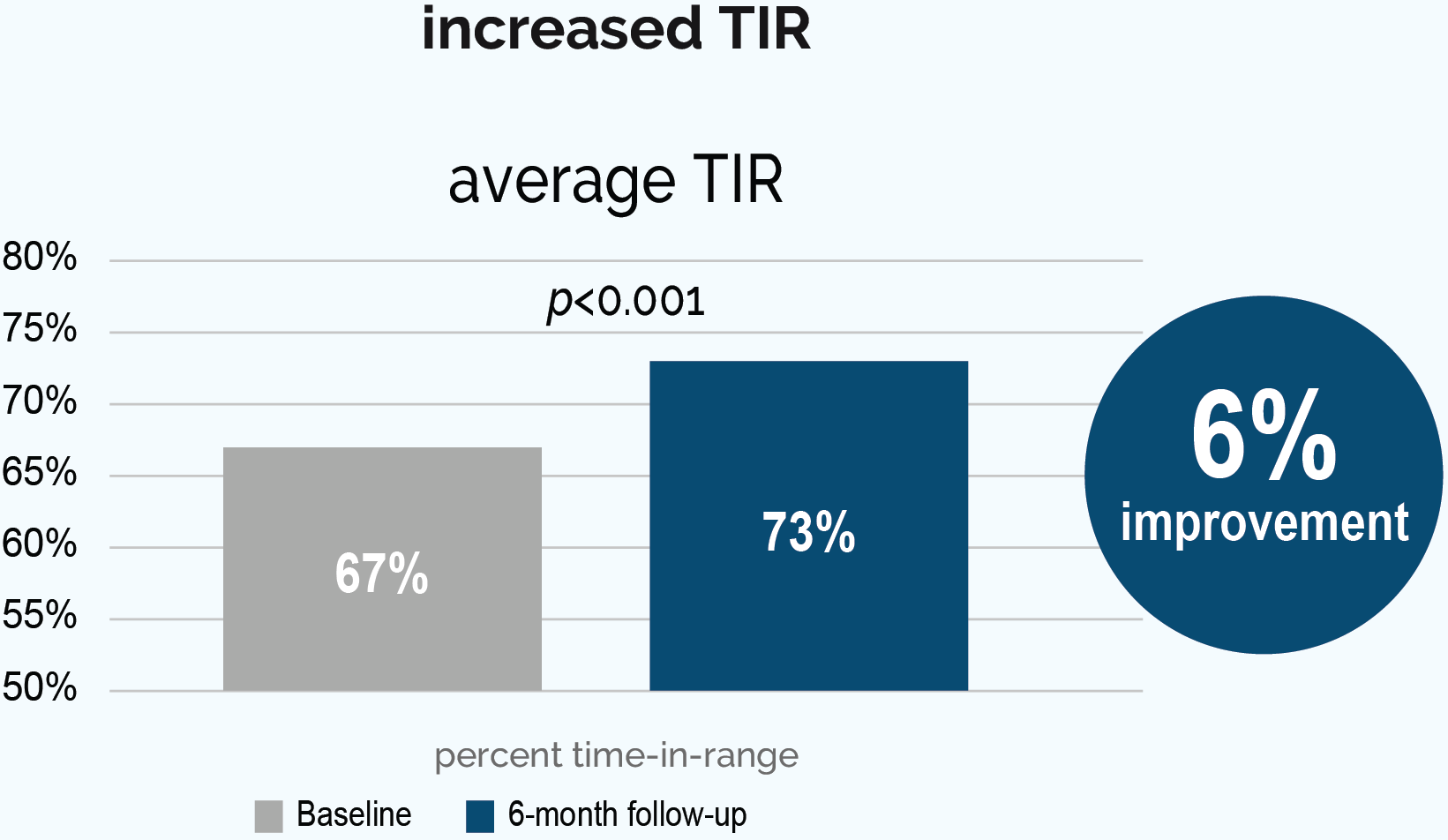
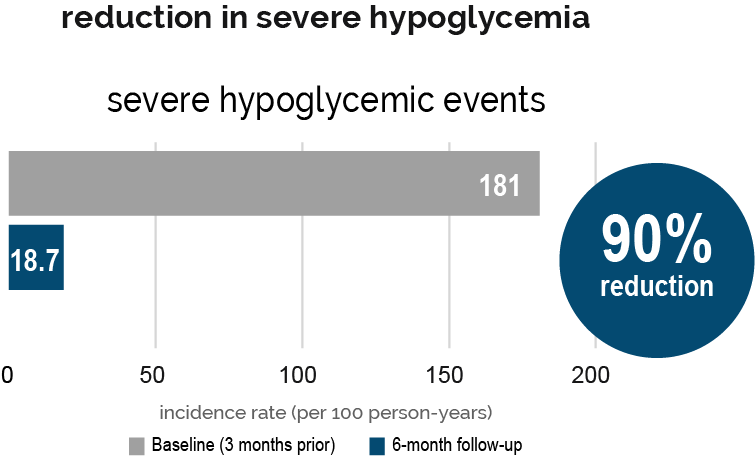
†Results obtained with the DIY Loop algorithm using a non-twiist™ device. DIY Loop was a predecessor to Tidepool Loop without the added guardrails, in-app education, and safety features added by Tidepool.
Study design: Approved by the Institutional Review Board of the JAEB Center for Health Research (ClinicalTrials.gov Identifier: NCT03838900), Lum et al conducted a prospective, observational, real-world study in 558 adult and pediatric patients with T1D (mean age 23) to evaluate the effectiveness and safety of the DIY Loop. Patients initiated the DIY Loop either on their own, or with community-developed resources, and used the DIY Loop algorithm with a non-twiist insulin pump over 6 months. After 6 months, 75% of patients studied achieved A1C <7.0% compared with 58% of patients at baseline.2
the opportunity for better quality of life


†Results obtained with the DIY Loop algorithm using a non-twiist™ device. DIY Loop was a predecessor to Tidepool Loop without the added guardrails, in-app education, and safety features added by Tidepool.
Study design: From an ongoing observational study evaluating glycemic control, adverse event rates, and patient-reported outcomes among patients with T1D, participants were recruited through online postings and packaging of the Loop RileyLink. Device data were collected via the Tidepool Mobile App, including available CGM data at enrollment, for the first 3 months using Loop. Patient reported outcomes evaluated diabetes distress, technology attitudes and problem solving, hypoglycemia fears and confidence, and sleep quality with validated survey. Baseline diabetes distress decreased after 3 months from 2.06 to 1.66 (n=254). Baseline sleep quality improved after 3 months from 6.82 to 5.39 as measured by the Pittsburgh Sleep Quality Inventory (n=201).
iiSure™ Technology—detects occlusions up to 9x faster than other AID systems3‡
Sound wave technology detects occlusions 2.5+ hours faster than Omnipod 5.
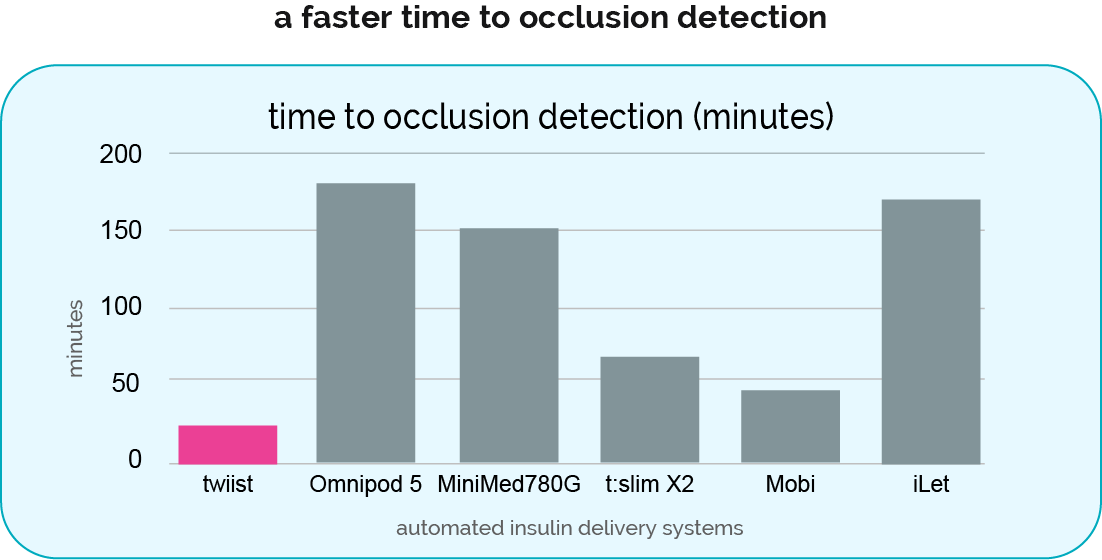
‡Calculation based on 1 U/hr basal rate and the following sources: Omnipod 5 Automated Insulin Delivery System. t:slim X2 Insulin Pump User Guide (AW-1011648_B; listed at 2 U/hr). Tandem Diabetes Care. Tandem Mobi System User Guide (AW-1012691_A). December 2023. MiniMed 780G System User Guide (M036461C001 2). iLet Bionic Pancreas System User Guide (LA000081_D).
REFERENCES
1. DEKA R&D Corp. DEKA ACE Pump System with DEKA Loop User Guide. February 2024. 2. Lum JW, Bailey RJ, Barnes-Lomen V, et al. A real-world prospective study of the safety and effectiveness of the Loop open source automated insulin delivery system. Diabetes Technol Ther. 2021;23(5):367-375. 3. Data on file. Sequel Med Tech, LLC. 2023. 4. Hood KK, Wong JJ, Hanes S, et al; 1001-P: Do-It-Yourself (DIY) Loop is associated with psychosocial benefits. Diabetes 1 June 2020; 69 (Supplement_1): 1001–P. https://doi.org/10.2337/db20-1001-P
Indications for Use
The twiist system is intended for the subcutaneous delivery of insulin, at set and variable rates, for the management of diabetes mellitus in persons requiring insulin, ages six and above. The pump is able to reliably and securely communicate with compatible, digitally connected devices, including automated insulin dosing software, to receive, execute, and confirm commands from these devices. The twiist system is intended for single-patient home use and requires a prescription.
Loop is intended for use with compatible integrated continuous glucose monitors (iCGM) and the twiist alternate controller enabled (ACE) insulin infusion pump to automatically increase, decrease, and suspend delivery of basal insulin based on iCGM readings and predicted glucose values. It can also recommend, and with the user’s confirmation, deliver correction boluses when glucose values are predicted to exceed user configurable thresholds. Loop is intended for the management of type 1 diabetes mellitus in persons six years of age and greater, and is intended for single-patient use. The twiist automated insulin delivery system is for prescription use only.
The simple bolus calculator, available when Loop is off, is indicated for use for aiding the user in determining the bolus insulin dosage for management of diabetes mellitus based on consumed carbohydrates, operator-entered blood glucose, insulin sensitivity, insulin to carbohydrate ratio, correction range, and current active insulin.
Contraindications
- Test blood glucose (BG) levels as recommended by their
healthcare provider - Maintain sufficient diabetes self-care skills
- See their healthcare provider regularly
- Demonstrate adequate carbohydrate-counting skills
- Have difficulty seeing
- Have difficulty hearing
- Have deficient cognitive capabilities
- Have physical impairments that would make
operating the device or iPhone difficult
“twiist,” “iiSure,” and the twiist logo are trademarks of Sequel Med Tech, LLC. “Tidepool” and the Tidepool logo are trademarks of Tidepool Project, a fully-qualified 501(c)(3) nonprofit organization.
© 2024 Sequel Med Tech, LLC. All rights reserved.
